In May 2024, Swiss Medtech published a guidance document on how to sell medical devices in Switzerland after the legacy deadline on 26 May 2024. This Swiss Medtech document targets both non-Swiss and Swiss manufacturers that have CE-marked medical devices under the Directives (MDD and AIMDD) which are not transitioning to the MDR.
Devices that can no longer be placed on the market but can be made available
Whether legacy devices can be placed on not on the market in Switzerland depends on their transition to the MDR.
Legacy devices which are not transitioning to the Medical Devices Regulation (MDR) can no longer be placed on the market neither in Switzerland nor in the EU after 26 May 2024. From 27 May 2024, manufacturers must disregard these devices. Devices placed on the market by 26 May 2024 can remain on the market and no sell-off provisions apply, meaning that there is no deadline to remove these devices from the supply chain.
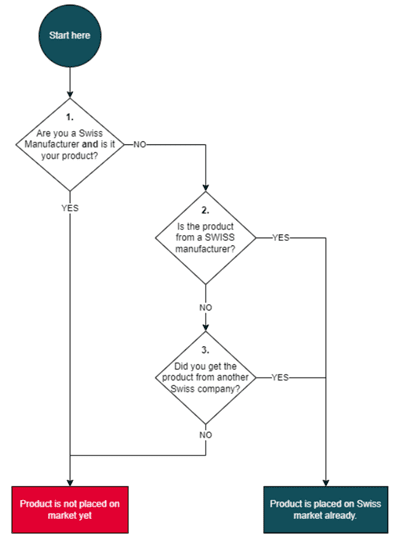
The Swiss definition of “placing on the market” corresponds to the interpretation of the European Blue Guide, which specifies that “the placing on the market is the moment in which the product is supplied for distribution, consumption or use for the first time”. The Swiss Medtech guidance presents a flowchart that manufacturers can use to understand if their product is considered as placed on the Swiss market or not:
Source: Swiss Medtech (2024)
Legacy devices transition to the MDR can be placed on the Swiss market
If a manufacturer filed an MDR application with a notified body within (for the legacy device or a device intended to replace it) and have an MDR compliant Quality Management System (QMS) within 26 May 2024, Switzerland recognises the extended legacy period (the CE certificate’s extended validity or prolonged transitional period) and the concerned legacy devices can therefore be placed on the Swiss market after 26 May 2024. Switzerland requires all non-Swiss manufacturers to have a Swiss authorised representative (except Lichtenstein-based manufacturers) and comply with the MedDO (the Medical Devices Ordinance).
Most importantly, manufacturers have to have a signed MDR agreement with a notified body by 26 September 2024.
Do you have any questions on how to enter the Swiss, EU or UK markets?
Simona Varrella
Regulatory Intelligence & Innovation
29.05.2024
Reference:
Swiss Medtech (2024) Placing on the Swiss Market or how to ensure you can sell-off all your legacy medical devices after 26.05.2024. Retrieved on 29.05.2024.
The information contained on obelis.ch is presented for general information purposes only, without obligation and it has been compiled with the utmost care to ensure it remains up to date. Nevertheless, Obelis Group cannot be held liable for the accuracy and completeness of the information published. Any reliance placed on such information is therefore strictly at the User’s risk.


