Without a mutual recognition agreement between the European Union and Switzerland, medical devices and IVD medical devices are subject to the Swiss legislation. Compliance with only EU Regulations (MDR and IVDR) is no longer sufficient to place medical devices on the Swiss territory.
Swissmedic and Swissdamed registration for economic operators
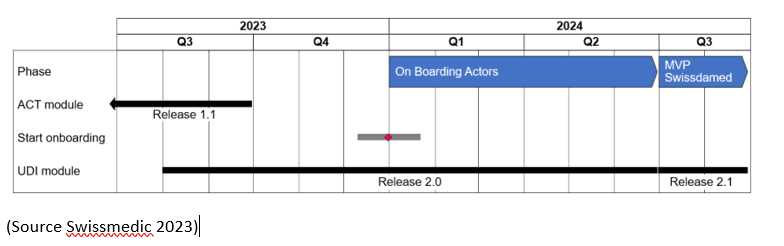
In accordance with the Medical Devices Ordinance and IVD Medical Devices Ordinance (MedDO and IvDO), Swiss manufacturers, importers, and authorised representative have to register with the Swiss competent authority. To fulfil this requirement, Swissdamed, the “Swiss Database on Medical Devices”, will become functional in 2024. Before its functionality, Swiss economic operators have to register with Swissmedic. Swissdamed will have two modules, the ACT module for the registration of companies and economic operators, and the UDI module for the registration of devices.
The roll-out plan is foreseen as follows:
What foreign manufacturers need to know to sell in Switzerland
The key information for all non-Swiss manufacturers is that compliance with the Swiss law is mandatory to place medical devices on the Swiss market. EU manufacturers and EU-compliant manufacturers also have to fully comply with the MedDO and IvDO. This means that all foreign manufacturers have to appoint a Swiss authorised representative (CH-Rep).
Interestingly, Switzerland is currenting working on a mutual recognition agreement with the FDA, the US Food & Drug Administration, meaning that FDA-approved medical devices might in the following years.
If you are a manufacturer of medical devices and want to know more about selling your products in Switzerland, contact us today.
Simona Varrella
Publications Department
17/08/2023
Are you interested in selling your medical devices in Switzerland? Obelis can act as your Swiss Authorised Representative and turn your Swiss journey into a success story! Contact us today.
References:
Swissmedic (2023) Registering economic operators (CHRN). Retrieved on 17/08/2023.
Swissmedic (2023) swissdamed – Swiss Database on Medical Devices. Retrieved on 17/08/2023.
The information contained on obelis.ch is presented for general information purposes only, without obligation and it has been compiled with the utmost care to ensure it remains up to date. Nevertheless, Obelis Group cannot be held liable for the accuracy and completeness of the information published. Any reliance placed on such information is therefore strictly at the User’s risk.


